Dr. Emmanuel Pameté
Development of high energy hybrid capacitors
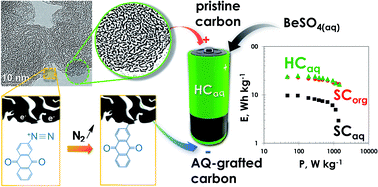
The limited specific energy and high cost of electrochemical capacitors is a main obstacle which slows down their large-scale implementation in new industrial segments, e.g., electric and hybrid vehicles. In order to enhance the voltage and consequently the stored energy, the available technologies are based on the use of organic electrolytes, either in an electrical double-layer (EDL) configuration with nanoporous carbon electrodes, or in a hybrid construction with an EDL positive electrode and a lithium (or sodium) intercalation/insertion carbon anode. As the stability window of the electrolyte might be dramatically altered by water impurities even in ppm amount, it requires in both cases to carefully dry the carbon electrodes and to construct the cells in moisture-free atmosphere, what substantially increases the cost of these technologies. Considering the environmental unfriendliness of organic electrolytes, new concepts of electrochemical capacitors based on aqueous electrolytes have emerged during the last decade. An interesting attempt involves the redox properties of quinones dissolved in strongly acidic medium (concentrated sulfuric acid) - so-called redox electrolyte, with activated carbon electrodes. However, due to shuttling of quinone species between the electrodes, the self-discharge of the cells is very high and the maximum voltage is only 1 V. To reduce the self-discharge, it has been proposed to graft the quinone moieties on the negative carbon electrode material. However, the latter did not operate exclusively as a battery-type electrode even after careful mass balancing; in addition, sulfuric acid imposed using expensive current collectors made of noble metal.
We used the diazonium chemistry to graft a large amount (16 wt.%) of anthraquinone moieties on a high active surface area micro-/mesoporous carbon. The two-proton-two-electron redox process on the grafted quinone molecules could be activated by a less acidic electrolyte with pH ~ 2 (namely, aqueous beryllium sulfate), which additionally made possible the use of non-noble metal current collectors (from stainless steel). Owing to its highly reduced micropore volume, the grafted material demonstrated a high capacity in a very narrow potential range, which enabled to use it as a negative electrode coupled with the pristine carbon as positive electrode of wide potential range, and to provide a hybrid capacitor (HC) which could be charged up to 1.6 V with a very good life-span. As a result, at a given power, the hybrid capacitor stored the energy at the same level as the traditional EDL capacitor made from the same EDL carbon electrodes in organic electrolyte.
Hence, this new approach represents an important path towards the realization of cost affordable and environmentally friendly capacitors. Such breakthrough should undoubtedly serve as groundwork for researchers and engineers to optimize the performance of electrochemical energy storage systems. For example, the concept could be further optimized by lowering the potential of the redox active materials (e.g., by an appropriate choice of the active molecule and electrolyte), while simultaneously implementing high capacitance EDL positive electrode materials.
Design of nanoporous carbons/ionic liquid electrolytes for high performance EDLCs operating at low temperatures
Electrical double-layer capacitors (EDLCs) based on conventional organic electrolytes, such as 1 mol L-1 TEABF4 in acetonitrile (ACN), operate efficiently in a wide temperature range, -40/+70 °C, unlike batteries which storage mechanism (redox processes) aggravates at low-temperature causing their performance fading. However, ACN is volatile and flammable entailing a risk to the environment and safety. The application of solvent-free ionic liquids (ILs) as electrolytes allows to avoid these concerns as well as to increase considerably the operational cell voltage, thus the resulting energy. EDLCs based on single ILs have been investigated at room and elevated temperatures, whereas due to their relatively high melting points, selected binary mixtures of ILs have been proposed for capacitors operating at low-temperature. Since ILs have greater viscosity than ACN-based electrolytes, mesoporous electrodes, such as graphite oxide have been implemented to enable efficient ion transportation. Nevertheless, these materials have relatively low density imposing low volumetric capacitance of the EDLCs.
We designed a strategy for the development of carbon/carbon EDLCs with IL electrolyte operating at low-temperature with high gravimetric and volumetric outputs. First, we formulated two types of IL electrolytes, a binary mixture of [EMIm][FSI]0.5[BF4]0.5 (σ = 12.1 mS cm-1 at 20 °C and 0.42 mS cm-1 at -40 °C) and a ternary one of [EMIm][FSI]0.6[BF4]0.1[TCB]0.3 (σ = 14.2 mS cm-1 at 20 °C, σ = 1.06 mS cm-1 at -40 °C and σ = 0.56 mS cm-1 at -50 °C). Both mixtures exhibited promising transport properties as well as a wide liquid range at low-temperature, till glass transition at around -90 °C. Next, we focused on the preparation of carbonaceous electrodes with a substantial share of mesopores and improved density.
Contact Information
INM-Leibniz Institute for New Materials
Campus D2 2, 66123 Saarbrucken, Germany
Tel: +49 (0)681-9300-402

©2017 by Pye. Proudly created with Wix.com